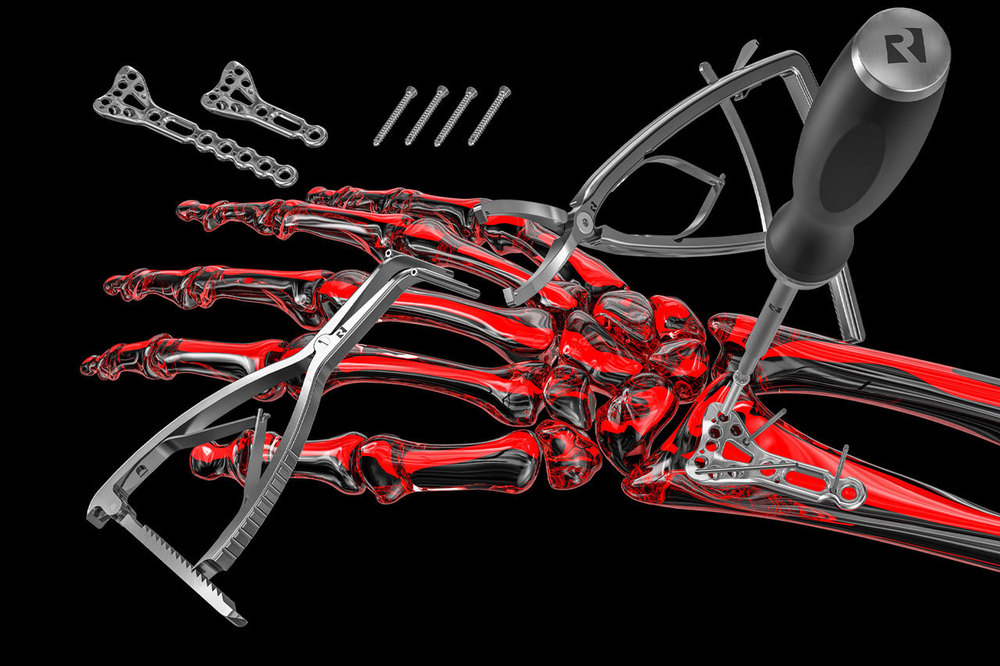
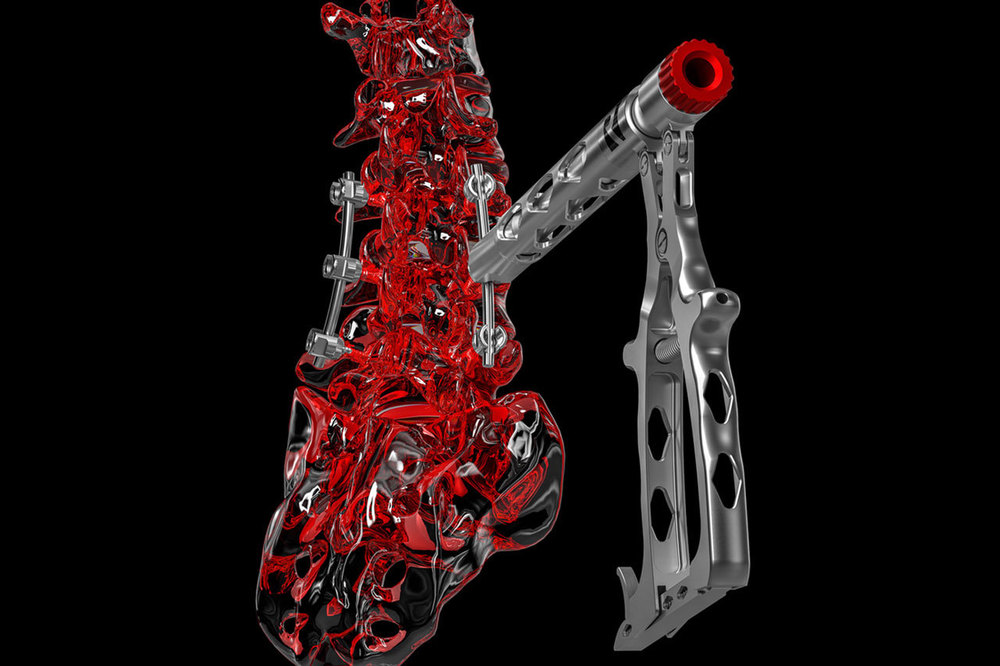
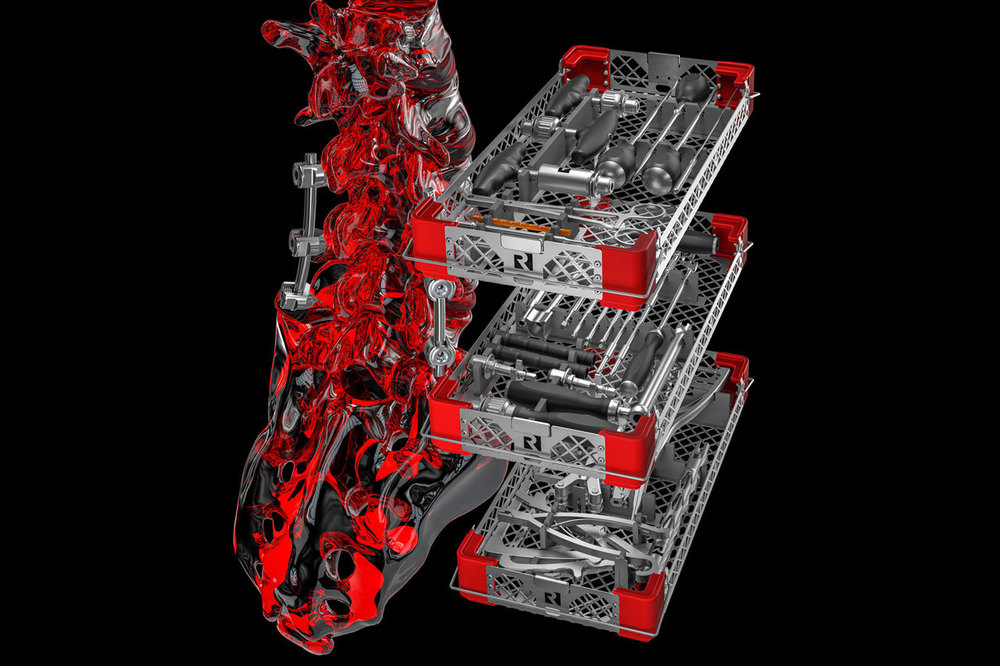
we are proud to provide our customers and partners our new certificate according MDR for quality management, EN ISO 13485:2016 + AC: 2016 - ISD 13485:2016, Valid until: 2024-06-05.
Rudischhauser is also FDA registered according FDA 21 CFR part 820 standard formulates the regulations for quality management systems of medical device manufacturers no.: 8010300