UDI, MDR and Co in sight
In addition to examples of how digitization can help to optimize processes and comply with regulations, there is valuable content from the area of "Unique Device Identification (UDI) for medical devices" and current updates on MDR/IVDR.
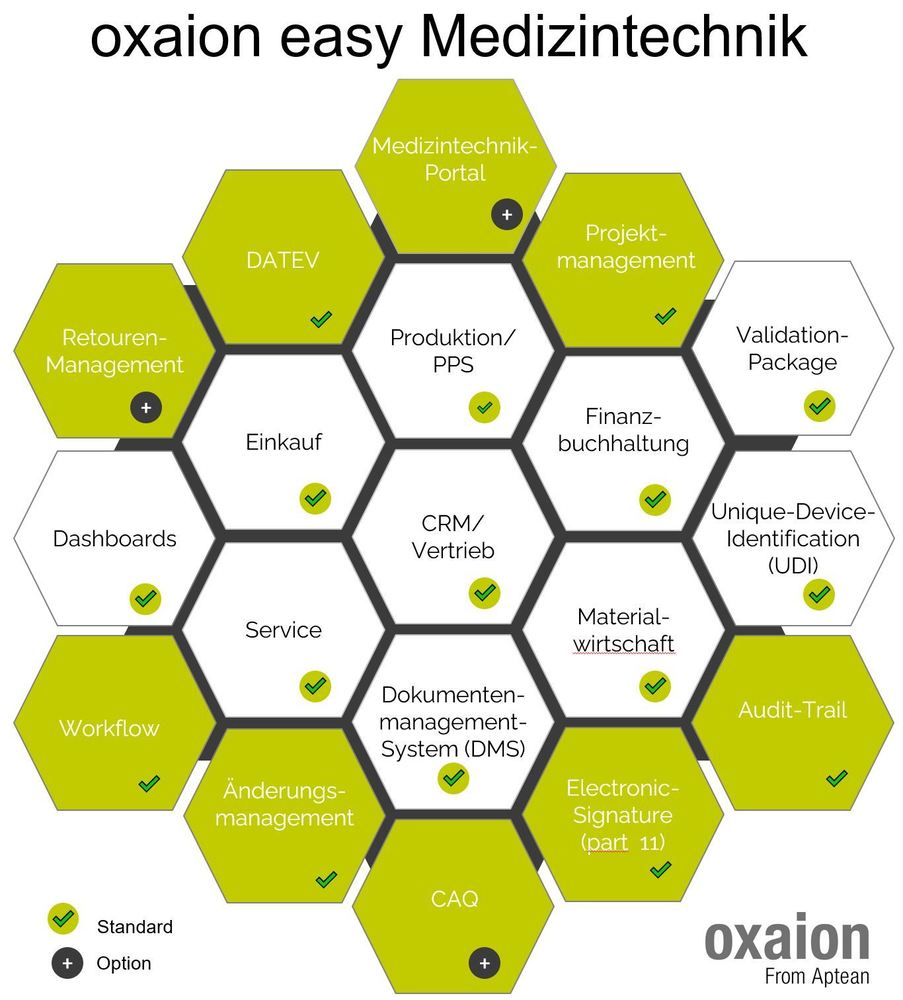
The extent to which digitization of medical technology helps to optimize processes holistically and at the same time comply with all regulations is illustrated by oxaion industry manager Jens Fröhlich using the example of Inpac Medizintechnik GmbH, a specialist in innovative medical technology packaging.
Daniel Rubisoier, Regulatory Affairs Specialist at TÜV SÜD, will then give a lecture on the general principles of "Unique Device Identification" (UDI) from the point of view of a notified body.
Sven Hertel, Manager Solutions Consultants at oxaion, shows how UDI is then best supported in practice by a modern ERP system.
Martin Schmid, Managing Director at encotec GmbH, then discusses the main challenges regarding the new EU regulations on medical devices (MDR and IVDR) from the perspective of an economic player. The Viennese consulting company encotec specializes in the development, approval and quality management of medical devices and in-vitro diagnostics.
You can now register free of charge at the Tirol location agency or directly at oxaion .
To the original article