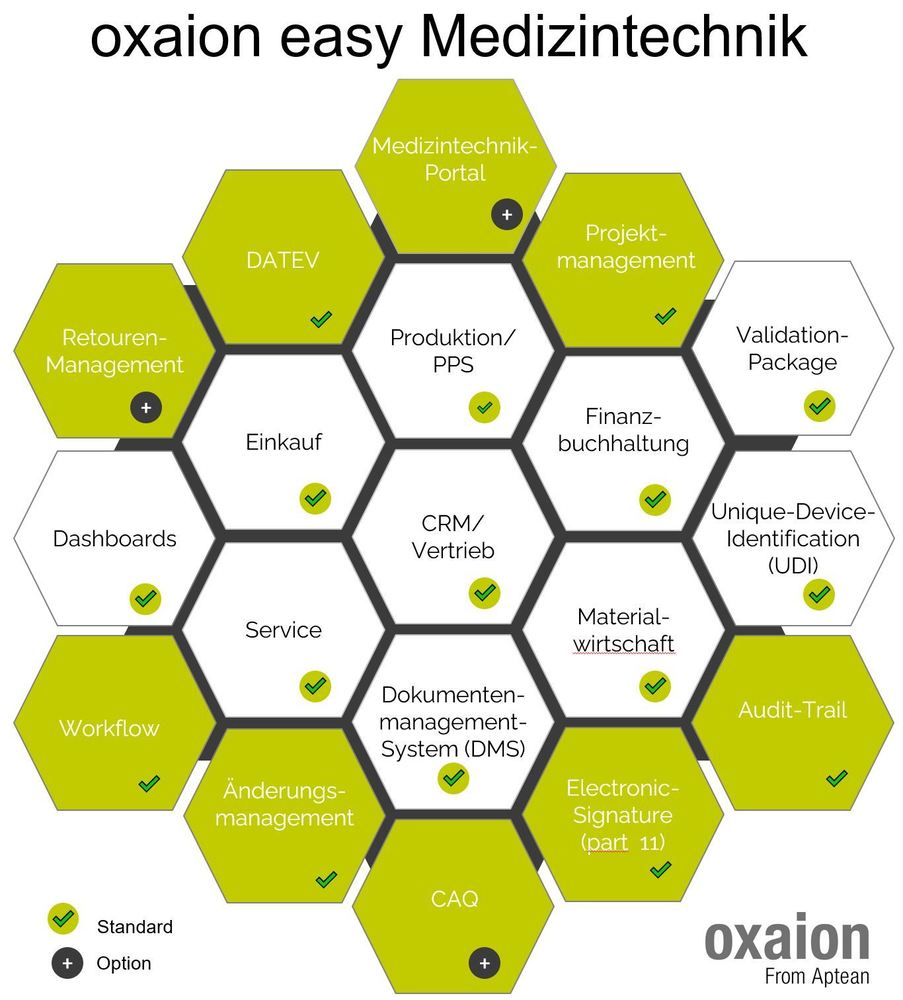
ERP and CAQ software solutions are playing an increasingly important role in software implementation projects in medical technology. Regulatory requirements are not of minor importance here.
Here we shed light on why an intelligent combination of both solutions plays an important role and how digitization can help you comply with regulations.
Two simple scenarios:
- The goods receipt in ERP is posted, a test order appears in quality control, which contains the characteristics to be tested for the article that has just been unloaded from the truck and received. After the successful inspection and approval, the goods receipt is approved and the items can be used for production or sale.
- The phone rings in service and the employee records a ticket with a complaint, the process is displayed in quality management and an assessment and risk analysis is started. A quick decision can be made here as to whether and which measures need to be initiated or whether a CAPA process needs to be carried out.
These two simple processes show that requirements should be met that must be taken into account when integrating ERP and CAQ. This applies, for example, to master and transaction data on both sides and permanent communication between the systems.
These are just 2 simple examples. Think, for example, of tests accompanying production / in-process controls or initial sample tests, we could list a number of other topics here.
In addition, there are regulatory requirements with regard to changing and releasing master data such as articles and parts lists through appropriate user authorizations and the electronic signature, as well as the change documentation via the audit trail.
On the CAQ side, it must be ensured, for example, that the test equipment to be used is approved or that the correct test interval is observed. Here, too, of course, user authorizations, the electronic signature or the audit trail for traceability play a role. When ERP and CAQ interact, these requirements are taken into account so that it cannot happen that a blocked test equipment is used.
At Aptean, with oxaion open and SYNCOS CAQ, we have two solutions in our portfolio that are already deeply integrated with each other and interactively exchange a large number of master and movement data. Comprehensive product and quality management reduces the risk of an interface discussion. Both solutions will continue to grow together in the future.
How would you like more information?
Find out more from us now!
Visit us at the Swiss Medtech Expo in Lucerne from September 14th to 15th, 2021.
To the original article