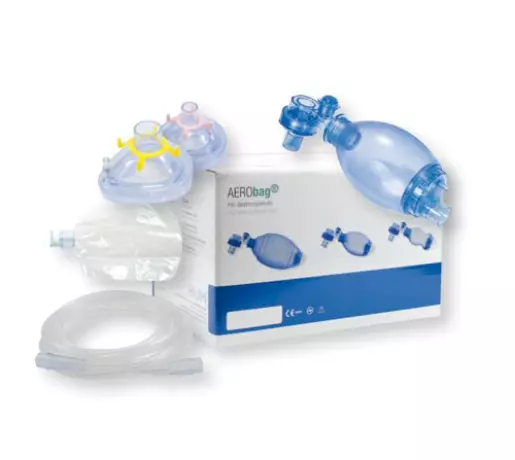
Gas supply - resuscitation bag AEROway resuscitation...
AEROway resuscitator silicone children Resuscitator silicone for children Bag 550ml O2 reservoir 2,6...
Portal and digital medical technology fair of the largest MedTech cluster in Germany

Gas supply - resuscitation bag AEROway resuscitation...
AEROway resuscitator silicone children Resuscitator silicone for children Bag 550ml O2 reservoir 2,6...

Emergency backpack - emergency bags
Emergency backpack - emergency bags Two-part interior with 5 modular pockets (color-coded) 2 side an...
Ihre Suche lieferte keine Ergebnisse, aber Sie können sich auf dieser Seite präsentieren
Mit einem Eintrag auf Weltzentrum der Medizintechnik platzieren Sie Ihr Unternehmen bei Google & Co. ganz weit oben und erreichen so jeden Tag mehrere Tausend potentielle Kunden aus der Medizintechnikbranche. Mehr Informationen gibt es auf der Firma eintragen - Seite.

During a natural disaster, it is imperative to have an effective system for emergency ventilation. There are a number of options for emergency ventilation, including manual ventilators, oxygen-powered demand valves, and exhaled gas ventilation. The best option is one that is easy to use, such as a wall switch. These devices are also easy to install. It is important to have an accessible switch and valve to initiate emergency notifications and ventilation operations.
Manual ventilation is a limited solution in the critical care environment, but there is currently no evidence for its long-term use. This approach can be inappropriate for many patients: they may be awake or sleeping, sedated or paralyzed, breathing spontaneously or weaning off a ventilator. In addition, the changing clinical presentations of ARDS often require shifting from minute ventilation to lung-protective strategies that place the patient at risk for auto-PEEP and other complications. A safe emergency ventilator can be a lifesaving solution in any of these circumstances.
Rapid design iterations enable the rapid development of a functioning prototype in one week. The two-level process involved additive manufacturing and testing 283 parts. In the second phase of the project, the authors used the same method to produce and test 75 functional prototypes. These ventilators were then tested in animals for two million cycles to ensure safety. The designs are publicly available under an open-source license. The team hopes to continue improving the safety of emergency ventilation for a long time.
While the technology has come a long way, a common problem remains: inadequate ventilation, a lack of expertise, and limited resources. Despite these problems, emergency ventilation techniques are still largely based on traditional centralized development models. Furthermore, the rapid changes in pandemics have made traditional, centralized processes ineffective. As a result, the authors provide a pragmatic open-source assessment framework that incorporates existing guidelines for emergency ventilation from Canada, the United Kingdom, and Australia. This open-source approach enables the design of an open-source emergency ventilator that can be tested to a two-million-cycle endurance test.
During an emergency, there are a number of different options for emergency ventilation. The first is a face mask, which uses a flexible plastic sheet to protect the face from infections. The second alternative is a self-inflating bag. A mask is usually made of clear plastic and should not allow for contamination. Regardless of the type of device, these devices are not only lightweight, but also easy to use. The portable version is available as an open-source project.
The CPR Medical Devices, Inc. is a manufacturer of patient-responsive emergency ventilation devices. The ventilators are based on a proprietary technology. The company's ventilators are sold to EMS and hospitals worldwide. The company's oxygen-delivering oxygen-filled bags are easy to use, and have no invasive components. They also offer accurate delivery of oxygen. These systems can also be used as an aid in a variety of different types of emergencies.
The DEVICE group is an informal association of faculty members who work in emergency medical equipment. Their team has come up with several concepts for improvement in emergency ventilation. It has created an oxygen-filled mask and has proof-of-concept 3D-printed COVID-19 swabs to test the ventilator's effectiveness. However, it is a necessary and crucial part of EMS. The DEVICE group is currently working on several other innovative ideas for emergency ventilation.
The company has been in the business of developing and distributing cutting-edge medical devices for years. Its products are designed to increase the survival rate of critical medical victims by improving the quality of the first treatment in harsh environments. Its SALI ventilator family is a simple mechanical system that is mated to an FDA-approved ventilatory circuit. The PREVAIL NY system is not meant to replace the FDA-approved ventilator. It is a rescue device for patients that have been intubated.
The Electromag group of companies develops and manufactures medical devices. It is the leading provider of ventilators for the critical care market. It offers a complete portfolio of modern solutions for hospital and homecare markets. In particular, the company has been a global leader in the development of portable oxygen analyzers for hospitals and non-medical applications. It also provides pulse oximeters and other medical equipment. Its products have been developed by a team of engineers who are experts in the field.
Become a digital exhibitor yourself in the online portal of the largest and best-known MedTech cluster region in Germany and inform the world of medical technology about your products and services as well as about news, events and career opportunities.
With an attractive online profile, we will help you to present yourself professionally on our portal as well as on Google and on social media.