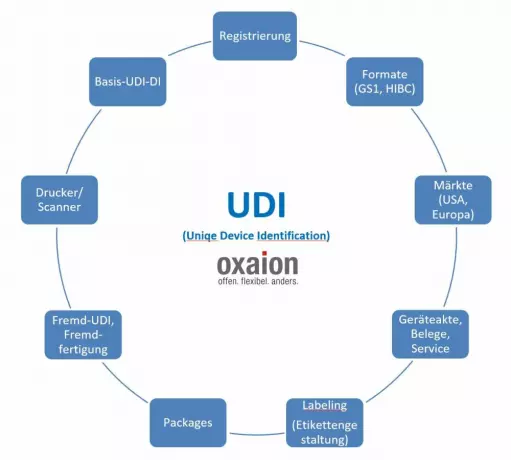
UDI as part of the business software in medical...
Not least because of the MDR, the UDI is developing into an essential component of overarching busin...
Portal and digital medical technology fair of the largest MedTech cluster in Germany

UDI as part of the business software in medical...
Not least because of the MDR, the UDI is developing into an essential component of overarching busin...

The notified bodies for the MDR and IVDR are the same, and are based in the United States and the European Union. They are designated by the British Standards Institute. The bodies are responsible for the assessment of medical devices and certifying their safety and effectiveness. The appointed authorities are independent and do not work for the FDA or any other regulatory authority. They also advise on the scientific evidence needed to assess a medical device.
The number of MDR and IVDR appointed authorities has increased significantly over the past 18 months, but there are still a large number of smaller regional Notified Bodies without much of a client base. Some of these have publicly stated that they will not apply for MDR and IVDR designations. Nonetheless, the Commission is moving toward achieving more MDR and IVDR notified bodies, even if their clients are small and unrepresented.
As for the MDR, there are many challenges facing manufacturers. First, the regulation was not intended to be as complex as it is today. The second directive will become effective in 2022, which means that the deadline for MDR and IVDR is a few years away. Consequently, implementing the directives will be a major challenge for the medical device industry. Secondly, a shortage of MDR notified bodies may prevent many devices from being certified in time for the MDR go-live date.
The MDR/IVDR will have a profound impact on medical device and diagnostic companies. To stay ahead of the competition, it is vital for manufacturers to have the proper business strategy in place. Contact ICON's experts for more information. These professionals can help you implement a sound business strategy and navigate the complex regulatory environment. These specialists are the perfect resource to guide you through the difficult road ahead. You will not regret your decision to enlist their help.
MDR and IVDR appointed authorities will be responsible for the assessment of medical devices. The new regulations will require NBs to have more extensive post-market clinical follow-up and post-market surveillance. The process is complicated and requires more investment than in the past, but it will save you money and time. It is crucial to get the correct guidance as soon as possible. The transition planning is essential. The next step is to identify the appropriate MDR and IVDR appointed authorities.
The MDR and IVDR appointed authorities will have different responsibilities. MDR will be the most comprehensive of all the two and will cover the requirements for both IVDR and MDR devices. The regulations will also regulate the quality of these medical devices. These experts will ensure the quality and safety of the medical devices. The MDR and IVDR are intended to prevent dangerous practices in the EU. Despite these differences, the aims of the two regulations are the same: To reduce the number of patients suffering from adverse effects, the MDR and IVDR will be more effective.
Become a digital exhibitor yourself in the online portal of the largest and best-known MedTech cluster region in Germany and inform the world of medical technology about your products and services as well as about news, events and career opportunities.
With an attractive online profile, we will help you to present yourself professionally on our portal as well as on Google and on social media.