
Contract Packaging and Sterilization for...
Contract Packaging and Sterilization of Medical Products From raw material to ready-to-...
Portal and digital medical technology fair of the largest MedTech cluster in Germany

Contract Packaging and Sterilization for...
Contract Packaging and Sterilization of Medical Products From raw material to ready-to-...
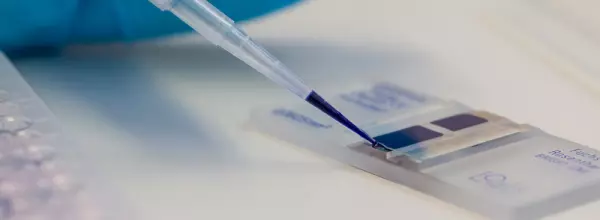
Biological Safety Testing of Medical Devices
Biological Evaluation of Medical Devices / Biocompatibility TPMD provides a highly sophisticated...

Test lab services for the medical device...
The components and devices we manufacture must stand up to unrelenting physical, biological and chem...

Getting ISO certification is an excellent way to improve your business's performance. Organizations that adopt the ISO 9000 quality management system are better equipped to meet the needs of stakeholders, as well as statutory and regulatory requirements. With ISO 9000 certification, you can be assured that you have adopted quality management practices that are recognized by stakeholders and customers alike. Here are some advantages of implementing the ISO 9000: 1. You'll be more consistent and able to meet regulatory and statutory requirements
Medical device manufacturers should consider ISO 14001: Environmental management standard. It can help them reduce their waste and energy consumption, and reduce their carbon footprint. It is based on the same structure as ISO 9001:2015, so if you have a certified quality management system, you should consider implementing ISO 14001 as well. It will also benefit you if you have a shared protocol with a medical device manufacturer. If you have a company that's already been ISO-certified, you can adopt the environmental standard and implement it with minimal effort.
Another benefit of ISO certification is that it is recognized globally. If your company has ISO 13485 certification, it means that the components that go into your medical devices are made to high standards. That's why your business can be more competitive. The FDA will investigate your company if they don't have ISO 13485 certification. And if you haven't received ISO 13485 certification, you'll need to make changes to comply with the standards. You'll need to recertify every three years or your certification will expire.
When it comes to medical devices, ISO certification is important. This helps ensure that your product is safe and efficient, while reducing your company's impact on the environment. Furthermore, ISO regulation ensures that your customers will have confidence in the quality of your products. It's important to make your business documentation as accurate as possible. By doing so, you'll have less rework in your processes, which will ultimately save you money in the long run.
ISO certification can be used for medical devices. Using the ISO standard makes it easier to reuse the certification documents for multiple countries. However, you need to ensure that you have a comprehensive understanding of the requirements in your country. This will help you avoid costly mistakes. The benefits of ISO Certification are numerous. Besides making your product safer, it will also protect consumers. The medical industry is the largest industry in the world. In fact, it is responsible for billions of dollars in revenue.
Companies with ISO certification can improve their profitability. It also provides a harmonized regulatory framework. It helps ensure that medical devices are produced according to the highest quality standards. And it serves as a proof of European Conformity for non-EU markets. With this, you can be sure of the safety of your products. It will improve your bottom line. If you want to get ISO certification, contact CMTC and learn more about the benefits.
Become a digital exhibitor yourself in the online portal of the largest and best-known MedTech cluster region in Germany and inform the world of medical technology about your products and services as well as about news, events and career opportunities.
With an attractive online profile, we will help you to present yourself professionally on our portal as well as on Google and on social media.