Regulatory secured - UDI is readable and correct
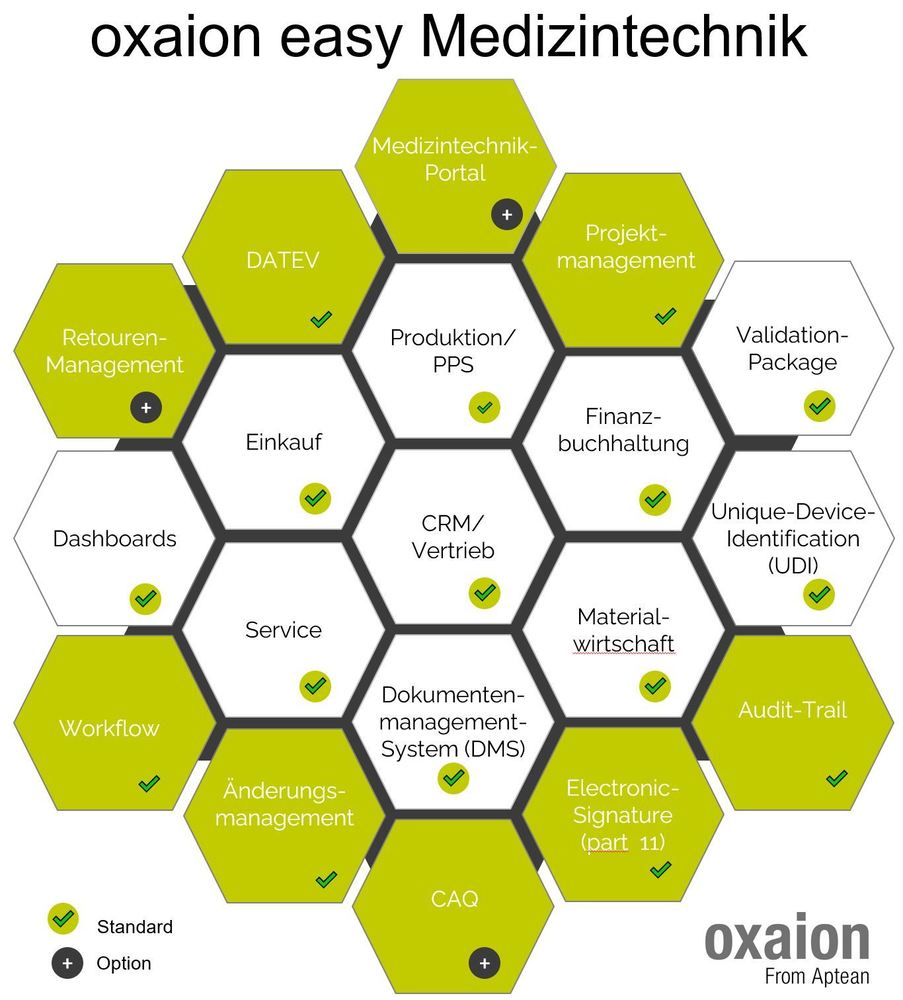
In the last 3 years we have expanded our ERP software solutions for medical technology, oxaion open and oxaion easy medical technology with many functions related to UDI and EUDAMED. The latest extensions relate to the creation of XML files for uploading to EUDAMED and the UDI transfer and documentation for commercial goods.
We have now completed an important milestone that will be of interest to many companies from a regulatory point of view: the verification and documentation of the UDI. In this way, everyone involved can be sure that the UDI is legible and has been created in accordance with the specifications.
We have created a joint integration with Elmicron GmbH, the specialist for UDI, barcodes and printing in the DACH region.
Essential UDI information, e.g. from production, is transmitted directly to Elmi-ScanLink Verify. Elmi-ScanLink Verify compares and checks the created UDI with the order data and informs the user of any differences. The report that is then automatically generated and stored is used for documentation.
Further information on Elmi-ScanLink Verify can be found at www.elmicron.de .
Here you will also find a lot of information about NiceLabel, barcode and UDI. Elmicron is also a recommended consulting partner of oxaion when it comes to UDI.
You can find more information about oxaion's UDI solutions here: https://www.oxaion.de/branchen/medizintechnik
To the original article