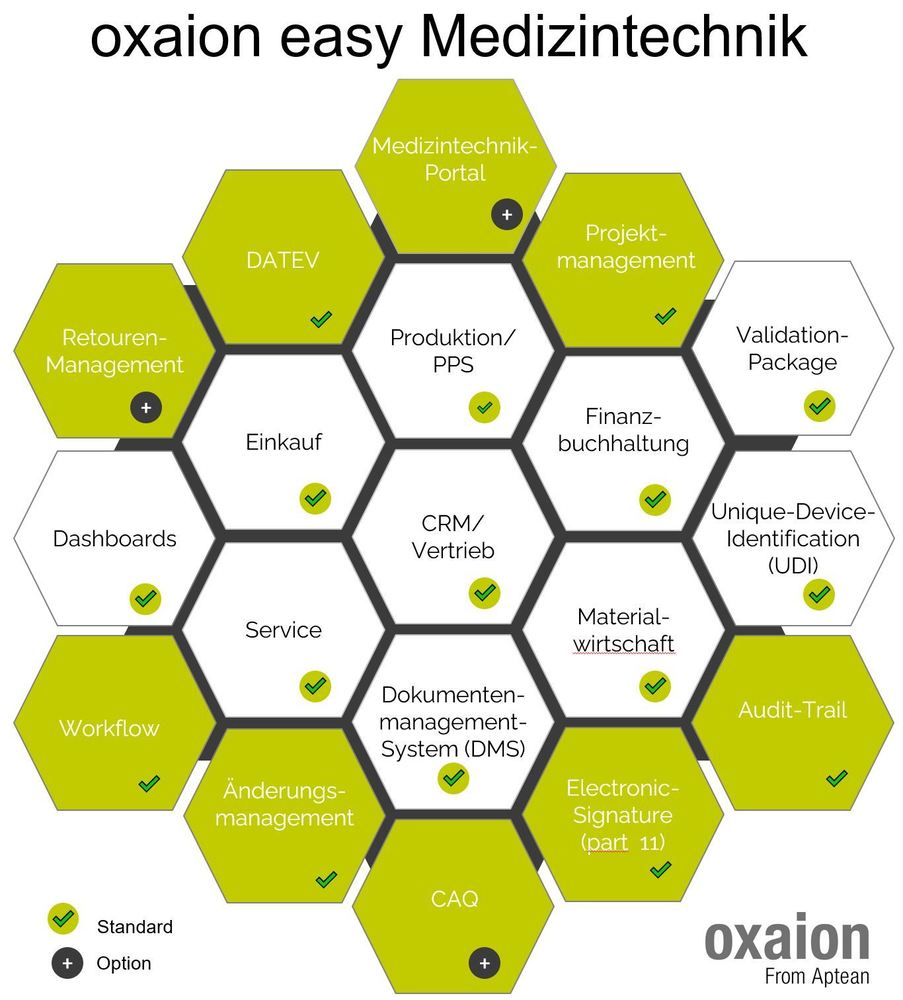
A medical technology company is looking for new business software. There can be different reasons for this. This can be technology, regulations or simply functional deficits. In the future, everything should be digital and modern ... A great challenge for companies. How do you find the "right" software now?
As a software provider, we usually experience two types of software selection: the company does it itself or looks for a consultant. There are good arguments for both variants, but we do not want to go into any further here. No matter which way a company goes, it is important to think about the basic requirements in advance. What does that mean?
Basic requirements are, for example, regulatory requirements, technical requirements or modules or departments that are to be implemented. It is of course also important to document the essential requirements from the individual departments. Topics are also important that may not be well represented today or are missing and which requirements should be mapped in the future. Of course, all processes should "run" better and more integrated.
Everything that is not known or not documented at the time of implementation will have to be clarified in the project. This often manifests itself in longer project times, increased costs and dissatisfaction on the part of the provider and customer.
In the regulatory environment of medical technology, some requirements must also be met, which, for example, require a validation of the computer system to be introduced or the processes to be mapped there. A so-called specification sheet is therefore a prerequisite for the above requirements. There are also some requirements for its form, such as proper indexing or numbering of the requirements.
Since this is not so easy to create in projects, we have created a validation package for smaller companies in which all relevant documents for validation are included.
At this point we would like to recommend our two whitepapers, which you can request from us free of charge:
Whitepaper: Digitization and regulations - IT in medical technology
Whitepaper: Cloud & Software as a Service for medical technology companies
Find out more now on:
www.oxaion.de/branchen/medizintechnik/
To the original article