Biological Safety Testing of Medical Devices
Biological Evaluation of Medical Devices / Biocompatibility TPMD provides a highly sophisticated...
Portal and digital medical technology fair of the largest MedTech cluster in Germany

Biological Safety Testing of Medical Devices
Biological Evaluation of Medical Devices / Biocompatibility TPMD provides a highly sophisticated...

ENDONEXT™ Bacterial Endotoxin Testing
The Evolution of Endotoxin Detection happens now! As part of any general pyrogen testing strategy...
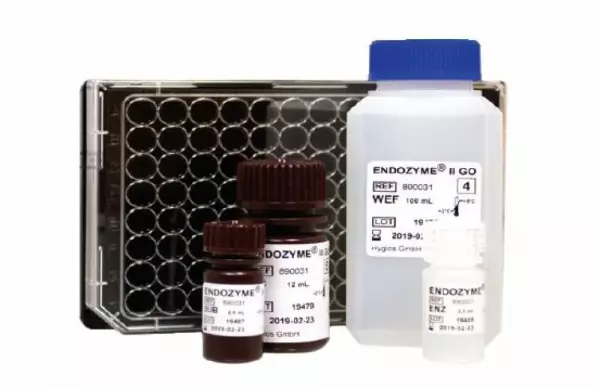
ENDOZYME® II GO (Endotoxin test)
MAIN BENEFITS: Fluorescence end-point assays in 96-well microplate format with methodologie...

Bacterial Endotoxin & Pyrogens: Monocyte...
TPMD is accredited and GMP certified for the determination of bacterial endotoxin (BET) and non endo...
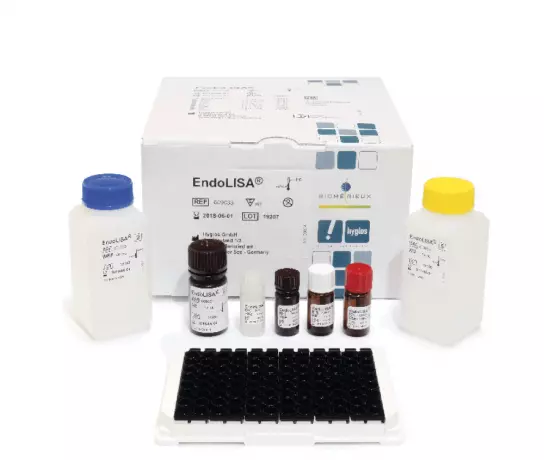
ENDOLISA® (Endotoxin detection)
Overcomes limitations of conventional methods, such as inhibition and enhancement...

Endotoxin test (as per USP 85 and Ph. Eur....
Endotoxins (ancient Greek endo: "inside", "within", toxins, "the poisonous...

Tests as part of the validation of instructions...
Manufacturers and distributors of medical deviceswhich are designed for reuse, must provide informat...

Tests as part of the validation of instructions...
In the same way as with processing of medical devices (cleaning and disinfection) the manufacturer o...

Endotoxins are harmful chemicals found naturally in the environment. They can be found in water, soil, and air. In addition, they can be released into the environment through human contact. Because of the importance of testing products for endotoxins, companies are required to use these tests to ensure that they are safe for use. These methods also add integrity to the products and make sure that their ingredients are free of contaminants. To determine whether a product contains endotoxins, the following steps are used.
LPS can persist in the environment for years, even without viable bacteria. This makes endotoxin tests essential for the safety of pharmaceuticals and medical devices. However, the LPS molecule itself is variable. To find out what the origin of the LPS molecule is, further studies are needed. This is why it is important to use endotoxin tests. In the meantime, LER testing can be used as a reliable way to ensure the safety of pharmaceuticals and medical devices.
Endotoxin tests are useful for manufacturers of medical devices. These tests can identify whether a particular product is safe for use. The most common method is to measure the concentration of endotoxins in a sample of blood. It is based on the color of the gel clot. The test involves using spectrophotometry to measure the concentration of the contaminant in the gel. The resulting color of the clot is then analyzed.
Aside from analyzing the toxicity of a substance, endotoxin tests can also be used to confirm the safety of medical devices. The FDA has guidelines for the LAL tests conducted on pharmaceuticals. These tests should be based on an enzyme extracted from the horse shoe crab's hemolymph cells. This method is based on the physiological reaction between endotoxins and amoebocytes. Amoebocytes are proteins that make up the cell walls of Gram-negative bacteria. These bacterial endotoxins damage the cells in a tissue culture and are released into the blood.
When endotoxins are present in medical devices, the bacteria in them may cause inflammatory responses and may even cause fatalities. Hence, the pharmaceutical industry has robust bacterial endotoxin testing workflows for detection of these pyrogenic components. They have replaced the rabbit pyrogen test with a newer test called the limulus amebocyte lysate. They are not only helpful for drug makers, but also for the general public.
Aside from the RSE, other endotoxin tests include the rabbit pyrogen test. This involves injecting a bacterial endotoxin into a live rabbit. The LAL test is an in vitro method, while the Control Standard Endotoxin Test is a bacterial endotoxin test. It is used for the detection of bacterial and fungal toxins.
Become a digital exhibitor yourself in the online portal of the largest and best-known MedTech cluster region in Germany and inform the world of medical technology about your products and services as well as about news, events and career opportunities.
With an attractive online profile, we will help you to present yourself professionally on our portal as well as on Google and on social media.