
Tests as part of the validation of instructions...
In the same way as with processing of medical devices (cleaning and disinfection) the manufacturer o...
Portal and digital medical technology fair of the largest MedTech cluster in Germany

Tests as part of the validation of instructions...
In the same way as with processing of medical devices (cleaning and disinfection) the manufacturer o...
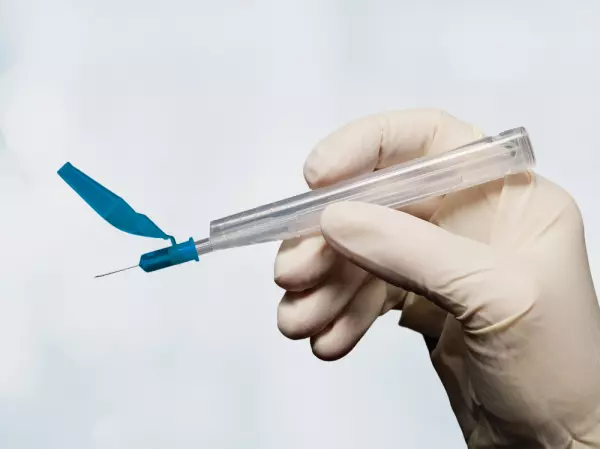
Case Study: Arterial Blood Collection Vessels
Whole blood samples are taken from patients using arterial blood collection vessels. The sample is t...

Contract Packaging and Sterilization for...
Contract Packaging and Sterilization of Medical Products From raw material to ready-to-...

Verification and validation of software in...
Our experience in a variety of safety-critical areas enables us to deliver an effective and efficien...

Tests as part of the validation of instructions...
Manufacturers and distributors of medical deviceswhich are designed for reuse, must provide informat...

Verification of IVD laboratory equipment, verification of laboratory products, verification of laboratory equipment, verification of analytical equipment, validation of IVD laboratory equipment, validation of laboratory products, validation of laboratory equipment, validation of analytical equipment
Verification of IVD laboratory equipment involves determining if the IVD instrument performs the tests as promised. The process of verifying the performance of IVD lab instruments ensures that they are produced as specified and that the reagents used are accurate and commutable. The verification process is acceptable for test procedures that are described in the product's package insert. In contrast, off-label use of IVD laboratory equipment requires a formal validation by a performing laboratory.
IVD instruments must meet AAMI/ANSI/IEC 60601-1-2 standards, which are more stringent than the requirements for laboratory equipment. It is therefore crucial for manufacturers of IVD lab equipment to identify the applicable standards and apply them from the design stage. The guidelines have made it easier to verify IVD instrument quality. Listed below are some of the important steps in ensuring IVD lab equipment meets regulatory requirements.
The regulatory requirements for IVD products differ from those for other medical devices, and are based on risk levels. In May 2017, the IVD Directive was replaced with the In Vitro Diagnostic Regulation (IVDR). The new regulation has expanded scope, reclassification, and increased documentation requirements. This can be advantageous for the healthcare industry and patients alike. However, it can also be costly. Whether the FDA's 510(k) or the FDA's PMA is required, IVD safety and quality will not be compromised.
The FDA has outlined several guidelines for IVD safety and effectiveness. The General Controls in the 1976 Amendments cover all IVDs. The standards include provisions related to adulteration, misbranding, registration, pre-market notification, and banned devices. It also covers good manufacturing practices. The FDA has a website that provides more information about IVD lab equipment. Further, it encourages manufacturers to disclose these guidelines, and it has been recommended that the European Union adopt this rule.
The FDA requires IVD manufacturers to demonstrate their devices' safety and effectiveness by conducting prospective clinical studies. The FDA also regularly requests clinical samples from the public. These samples help assess the clinical validity of the new device. They are expressed as clinical sensitivity, specificity, and agreement. The tests should be based on data from patients with similar medical conditions. In some cases, the FDA also requires a placebo to assess the safety of the drug.
The FDA has issued guidance for IVD manufacturers that label their devices as "Research Use Only" or "Investigational Use Only" (RUO). These products may include reagents, instruments, and systems used for research purposes. This guidance does not address the legal status of such tests. In fact, it covers only the pre-market approval of the product. The FDA also regulates the IUO test results.
Become a digital exhibitor yourself in the online portal of the largest and best-known MedTech cluster region in Germany and inform the world of medical technology about your products and services as well as about news, events and career opportunities.
With an attractive online profile, we will help you to present yourself professionally on our portal as well as on Google and on social media.