
1 - 9 by 9 results


Repair/MTK on Spacelabs long-term blood pressure...
Nispel GmbH tests and repairs your long-term blood pressure monitors according to the manufacturer&#...

Quality-verification processes for the medical...
No industry faces a higher mandate for quality than medical device manufacturing. At ADMEDES, we und...
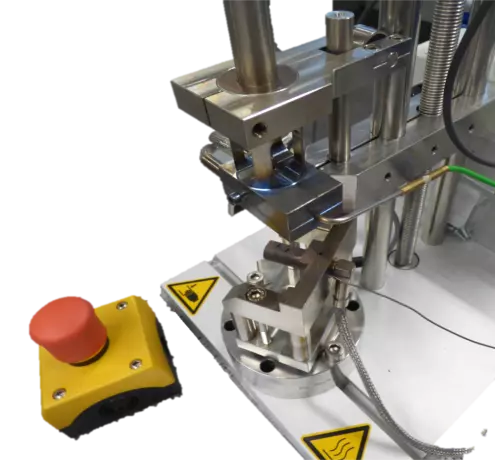
With the SOGA crimping press to the optimal result: The perfect hot crimping is a complex process...

Safety checks and DGUV4 tests We test your devices according to DIN VDE 0751 / DIN EN 60601-1 / D...
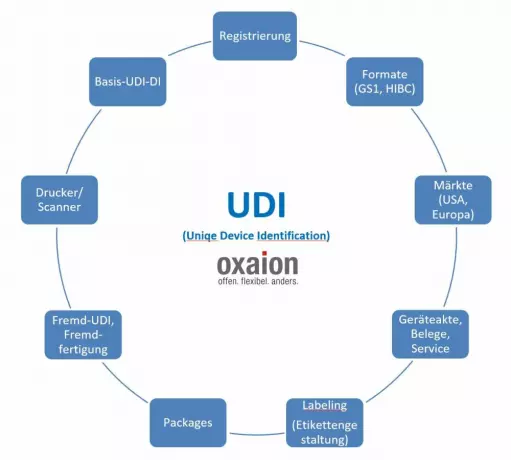
UDI as part of the business software in medical...
Not least because of the MDR, the UDI is developing into an essential component of overarching busin...

Assembly and final inspection of medical...
Assembly and final inspection of medical products Eurofins Inpac Medizintechnik GmbH assemb...
1 - 7 by 7 results
Quality control

There is no universally accepted process for quality control. It can be a difficult task to implement, especially since it depends on the industry and products. For example, a manufacturing firm producing food and drug products will have a process for ensuring that the labels on these products are correct, and it may also use feedback loops and chemical processes to monitor the process. However, in many cases, quality control is still not the same as quality assurance.
As a result, many manufacturers are finding it hard to meet the stringent requirements of quality control. This is especially the case for medical devices, which are often very expensive. As a result, many manufacturers are turning to advanced statistical algorithms to help them prevent production issues. With predictive analytics, companies can even anticipate possible production problems and avoid them before they happen. In this way, they can better meet their customers' needs and stay ahead of regulatory agencies' standards.
Medical device manufacturers need to ensure that their products are safe and reliable. A comprehensive quality control process is essential to avoid errors. Whether the medical device is a medical instrument or a pharmaceutical, it must have an accurate and consistent level of safety. For this, manufacturers need to adhere to strict regulations and abide by regulations. With these factors in mind, predictive quality analytics are crucial to medtech companies' success. This technology combines statistical algorithms and data from across all operations.
Medical device companies must document quality processes from the beginning of product development. Their quality management plans, working standards, and standard operating procedures must be thoroughly documented. This documentation process can be simplified by an enterprise quality management system (EQMS). It helps streamline the compliance activities and create smart links between all the required documents. If you're a medical device manufacturer, implementing a QMS is essential to a successful business. It is vital for the success of your company.
An organization must have a quality management system (QMS) in place from day one. The key to success is to keep improving it. The quality management system has a strong foundation, and medical devices will become more complex in the next few years. A successful QMS will help your company meet the regulatory standards. So, what are you waiting for? Get started today!
As an employee, you must be responsible for the quality of the products that you make. The company must be willing to accept customer complaints and comply with their standards. There are no shortcuts to ensuring the quality of your medical device. By using a QMS, you can reduce your costs and improve your products. You will also be able to improve the safety of your products and increase customer satisfaction. If you don't want to hire an QMS, you can take advantage of a conversion course.

















