
Implants and screws for the maxillofacial, orthopedic and trauma medicine area Surface anodized...
Portal and digital medical technology fair of the largest MedTech cluster in Germany

Do you have a product in mind? We can make it! Starting with the development / construction, we are...

CAD construction / development of medical...
CAD construction / development of medical products CAD has supplanted the drawing board and many...

Production of your OEM products - surgical...
Production of your OEM products - surgical instruments 100% Made in Germany OEM manufacturing...

Milling technology / milled parts - contract...
Milling technology / milled parts - contract manufacturing for medical products Milling refers to...

Orthopedic Products - Inserting Tools
The product range of cutting tools in the field of orthopedics includes: Phillips blades...

IVD Engineering Solutions - Custom Solutions
DIALUNOX Engineering Services - You have the Idea - we develop your product Custom Solutions D...

Modern diagnostic analysis methods require continuously smaller sample volumes. It brings advantages...

Higher quality finishing / finishing processes...
Higher quality finishing / finishing processes of surgical instruments / implants Refining or ref...

Production / contract manufacturing of medical...
Production / contract manufacturing of medical products on behalf of customers Since our foundati...

Research & development in the field of...
Do you have a product idea in the field of medical technology and need support with development?...

Turned parts, micro-turned parts, milled...
Drehen, Fräsen, Schleifen HIPP medical processes materials to turned parts and milled part i...

Medical technology industrial design
Medical technology Industrial design is an integral part of our product design process. We strive to...
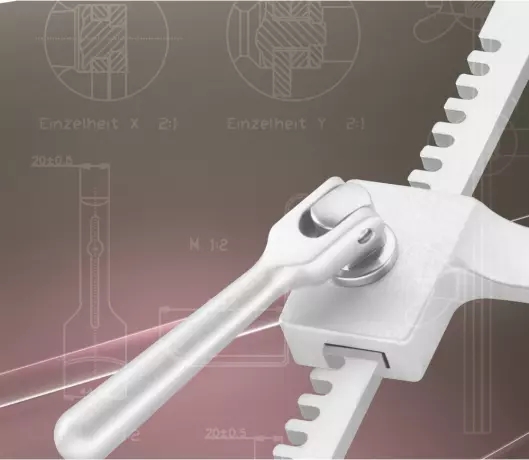
Our speciality: custom-made products You have a new idea or need an instrument solution for speci...
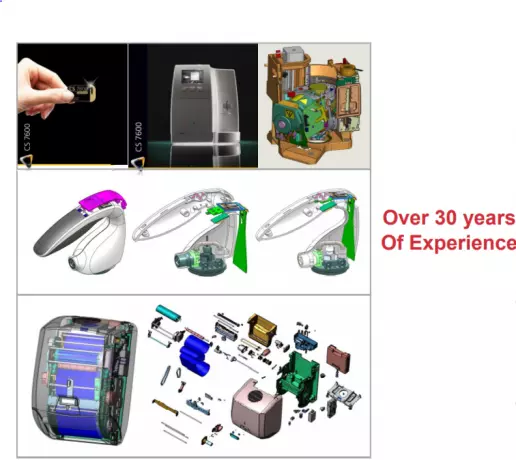
Medical technology engineering & development
Innovative and creative product design is vital to any successful product. For us it is important to...

Product development, prototyping or the manufacture of technical series are different from the pro...
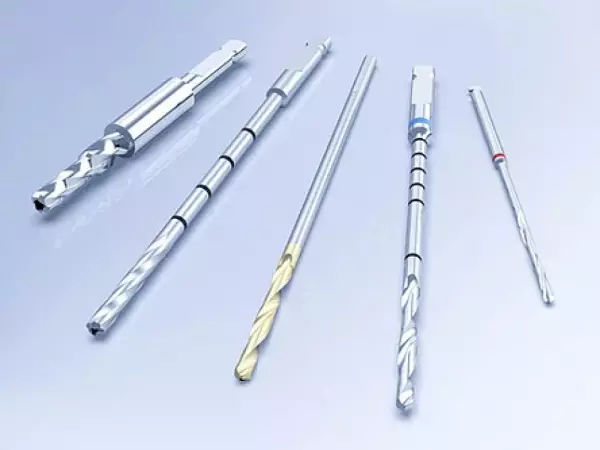
Orthopedic Products - Cutting Tools
The product range of cutting tools in the field of orthopedics includes: Bone drill Cu...

Orthopedic products - Navigating tools
Customized tools for navigating the drill holes and saw cuts

Healthcare & Medical assemblies
We are the reliable partner covering all areas from conception to (sterile) packaging of the finishe...

Healthcare & Medical Advanced Consumables
We produce smart medical consumables with added value. Added value means optimized and expanded prod...
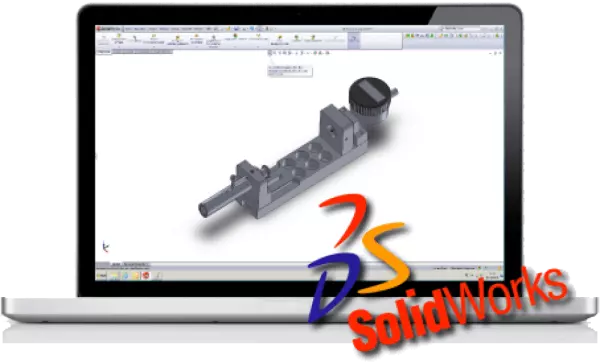
Contract manufacturing of precise turned...
Contract manufacturing of precise turned and milled parts for medical technology perame...

Custom-made products for medical technology
Or do you need a custom-made product? At Bumüller we develop and manufacture for you in-house. We wo...

Décolletage & CNC turning / parts - contract...
Décolletage & turning - contract manufacturing for medical products In classic turning...

Cnc turned-milled parts for medical technology
We manufacture to order according to customer drawings. Production according to sample parts is...

Custom made medical devices are devices that are not standard products. They are ordered under a written prescription from an authorized professional. For example, a Medical Doctor may request a specific device for a patient. The medical practitioner will describe the specific requirements, such as the design, anatomy, and materials. The manufacturer of the device must follow these guidelines, which can be a bit tricky for a small company. But, if the device is of a high enough quality and cost, it could make the difference between a high-quality product and a low-cost device.
The manufacturing of a custom-made medical device can be challenging, but the benefits of working with a trusted manufacturer are significant. While AM can produce a variety of shapes, materials, and features, the process is complex and requires expertise. In order to be effective, the manufacturer should take care to understand clinical data. Segmenting relevant parts from a DICOM file is an important part of the AM process. The data must be converted into the necessary CAD or stereolithography format. The clinicians and engineers will work together on the design and customization effort, but there are common pitfalls to avoid.
During the design process, a manufacturer must ensure that the design and construct of a custom medical device are compliant with regulatory requirements. This means eliminating risks as much as possible, ensuring adequate protection against risks, and letting users know about residual risk. It's important to consider the chemical, physical, and biological properties of a medical device to avoid infection or microbial contamination. The manufacturer should also consider radiation and other environmental conditions to ensure that the device is safe to use.
The manufacturing process for custom medical devices is more complex than for mass-produced products, so manufacturers must get a manufacturer's, importer's, or wholesaler's license before selling to consumers. While they are generally more expensive than mass-produced items, the FDA and NMPA do recognize their uniqueness. However, custom-made medical devices still have to go through standard market clearance and approval processes to ensure they're safe and effective.
In addition to having a unique design, a custom medical device must also be made in accordance with the written directions of a health care professional (HCP). The manufacturer must provide a declaration that contains data that allows the device to be identified and be used by the patient. It must also state that the device is intended solely for the patient. It should include the name of the patient, the name of the medical practitioner who issued the prescription, and the name of the clinic.
A custom-made medical device is produced on a written prescription. Its manufacture is unique to the patient's specifications. A custom-made device is typically more costly than mass-produced products, and the FDA may refuse to accept it if the manufacturer does not have the necessary certification. A physician should also carefully evaluate the custom-made device's performance after the trial period is over. It should also be durable, fit the patient, and be suitable for use.
Become a digital exhibitor yourself in the online portal of the largest and best-known MedTech cluster region in Germany and inform the world of medical technology about your products and services as well as about news, events and career opportunities.
With an attractive online profile, we will help you to present yourself professionally on our portal as well as on Google and on social media.