
oxaion easy medical technology - the business...
Smaller companies in medical technology in particular are feeling the pressure exerted by the new DI...
Portal and digital medical technology fair of the largest MedTech cluster in Germany

oxaion easy medical technology - the business...
Smaller companies in medical technology in particular are feeling the pressure exerted by the new DI...
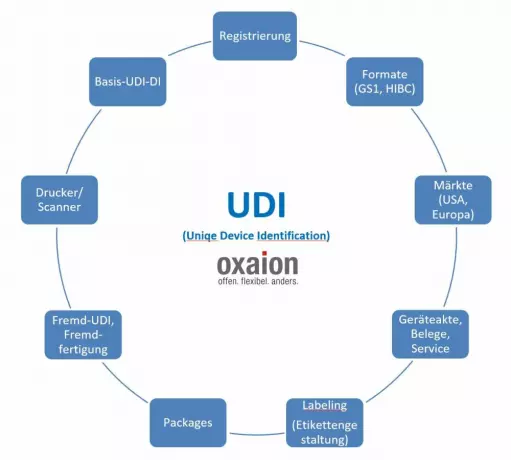
UDI as part of the business software in medical...
Not least because of the MDR, the UDI is developing into an essential component of overarching busin...

UDI database solutions help manufacturers comply with UDI regulations. These identifiers are a series of characters created by using internationally accepted standards. UDI helps companies identify specific medical devices on the market and facilitates their monitoring by applicable authorities. UDI is required for all medical devices that are subject to the MDR and IVDR, the new European directives. The GDSN database is managed by the European Commission and has a central database of UDIs for all types of products.
UDI database solutions are crucial to the success of medical device manufacturers. The UDID system has been implemented globally for all classes of medical devices. An independent regulatory body must monitor the system to ensure compliance and uniformity. Although the UDI system was first introduced in the US six years ago, it has not yet been adopted globally in all countries. As the system evolves, manufacturers must plan accordingly for the changes in regulatory requirements and technology.
UDI database solutions are crucial to the success of the UDI system. These solutions can provide comprehensive, reliable and timely UDI data and help manufacturers comply with UDI regulations. For example, Reed Tech's SingleSource (tm) solution enables companies to manage UDI data throughout the product lifecycle. This solution has multiple UDI channels for the US FDA, China NMPA, and South Korea MFDS. Additionally, it supports staging for EU EUDAMED.
UDI is embedded in the medical device, IFUs, and packaging. It should be unique for singularly packaged devices and not for devices that are sold in multiples. Different UDIs should be assigned to different types of packaging for different purposes. Similarly, the number of devices packaged together in a package must have different UDIs. Moreover, a new UDI must be generated every time a product undergoes a change.
UDI is a requirement for medical devices. It was first introduced by the US FDA and has been adopted by regulatory agencies around the world. Its main objective is to improve patient safety and improve the supply chain management of medical devices. Its UDI is an encrypted, unique identifier that can be used to identify the product. It should be printed on the packaging. If the device is on a physical medium, the UDI should be recorded in the case of the corresponding item.
UDI database solutions are an essential part of a regulated medical device. They can help companies comply with UDI regulations and ensure that their products are safe. A UDI database solution is a vital element of medical device regulation. With UDI, manufacturers will be able to track the performance of their products. This allows them to create a better business model. Further, a UDI database will help them track and monitor their devices and improve their safety.
Become a digital exhibitor yourself in the online portal of the largest and best-known MedTech cluster region in Germany and inform the world of medical technology about your products and services as well as about news, events and career opportunities.
With an attractive online profile, we will help you to present yourself professionally on our portal as well as on Google and on social media.