LRE Medical GmbH company portrait
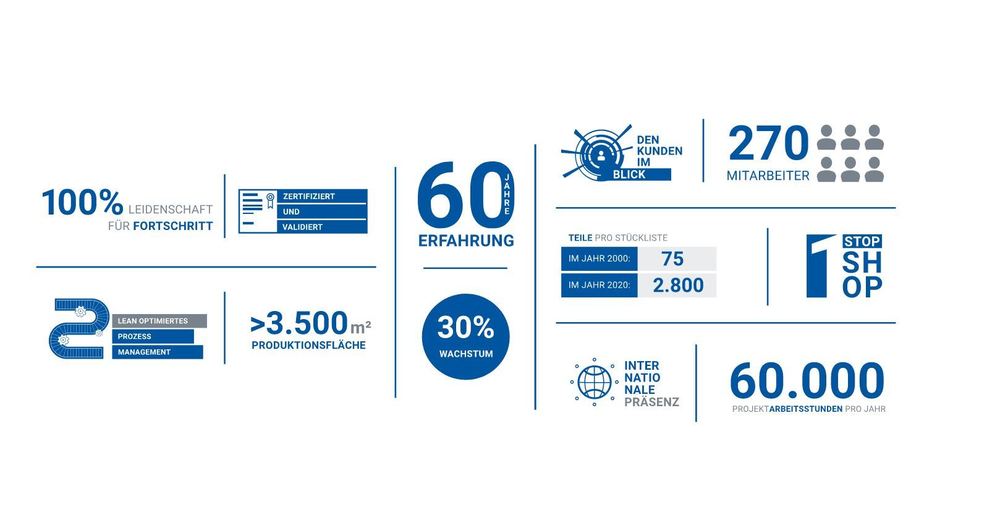
LRE Medical GmbH – established 1961 in Munich – develops and manufactures instrumentation for the diagnostics industry (Large corporations, medium sized companies and start-ups) in the following markets.
- In Vitro Diagnostics
- Life Sciences
- Analytics
- MedTech
LRE has grown to a leadership position through the application of innovative solutions to meet critical client needs.
Our headquarter is in Nördlingen, Germany (210 employees). Product development is in Munich, Germany (70 Employees, in addition to our permanently employed staff we cooperate with contractors and temporary staff).
Product development
Our skilled team ensures successful and beneficial product development programs
- System Development
- Risk Management
- Electronics Hardware Design
- Software Development and SW Testing
- Optics Design
- Mechanical Design
- Usability
- Prototyping (Electronics, mechanics)
- EMC Testing Chamber (for orienting measurements)
Process development
Smooth and cost effective transition from product development to manufacturing through early involvement of the manufacturing specialists.
- Design transfer from in-house and externally developed products to serial manufacturing
- Process design according to GMPc guidelines
- Design and implementation of efficient in-process controls
- Design of supporting tools and fixtures
- Process analysis
- Process adjustment programs
Manufacturing
Flexible and modern manufacturing facility with state of the art processing methods:
- Optimized conditions for highest quality assembly of medical instrumentation: Printed circuit board assembly, sub- and device assembly, testing (In circuit- functional, AOI, AXI, component traceability system) and labeling.
- Product focused manufacturing work cells with all the needed resources for a particular product readily available in special areas on our production floor
- LRE operates in a lean environment