




CleanControlling Medical GmbH & Co. KG
CleanControlling Medical GmbH & Co. KG company portrait
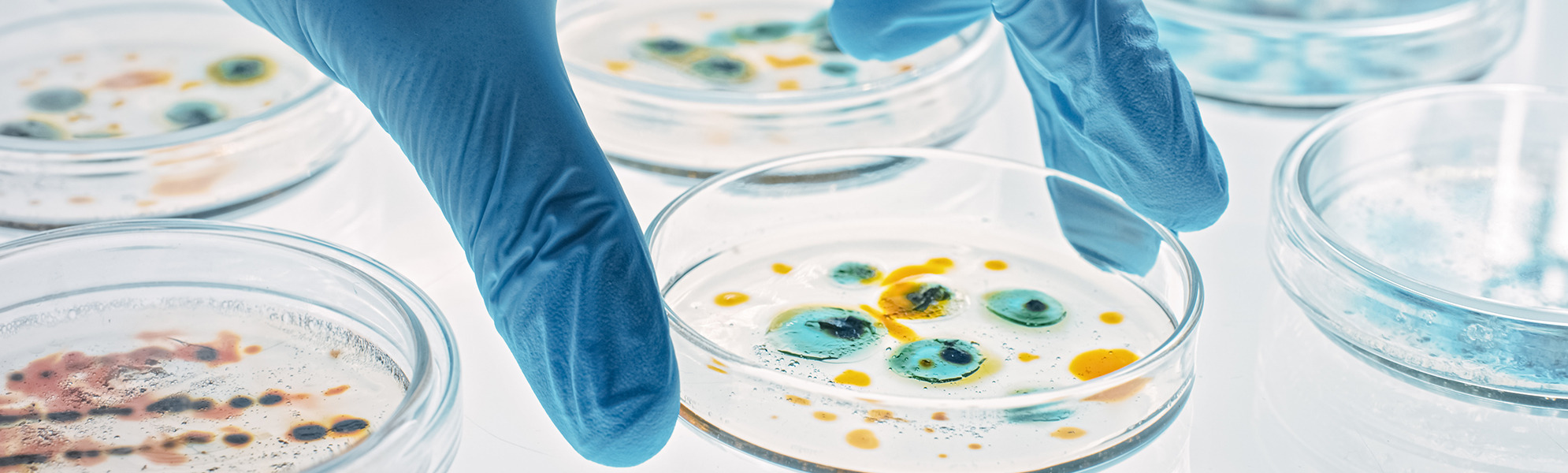
CleanControlling Medical GmbH & Co. KG offers a comprehensive portfolio of laboratory services as a GLP-certified, DIN EN ISO / IEC 17025 accredited and ZLG-approved test laboratory for biological and microbiological-hygienic testing of medical devices.
The range of services includes tests to determine the biocompatibility according to the amendment of DIN EN ISO 10993-1, tests for the validation and monitoring of cleaning processes, determination of particulate cleanliness, tests within the validation of reprocessing and sterilization instructions, life tests as well as tests for determination the chemical surface cleanliness.
In addition to the biological and microbiological-hygienic competences, there is a special focus on tests for the determination of particulate cleanliness. Due to the company's origin of CleanControlling GmbH in the area of "Technical Cleanliness" in industry and automotive, CleanControlling Medical GmbH & Co. KG relies on the comprehensive know-how from different methods for analysis, typification and characterization of particles as well as on the profound knowledge of cleanliness in industrial production processes.
Biological tests
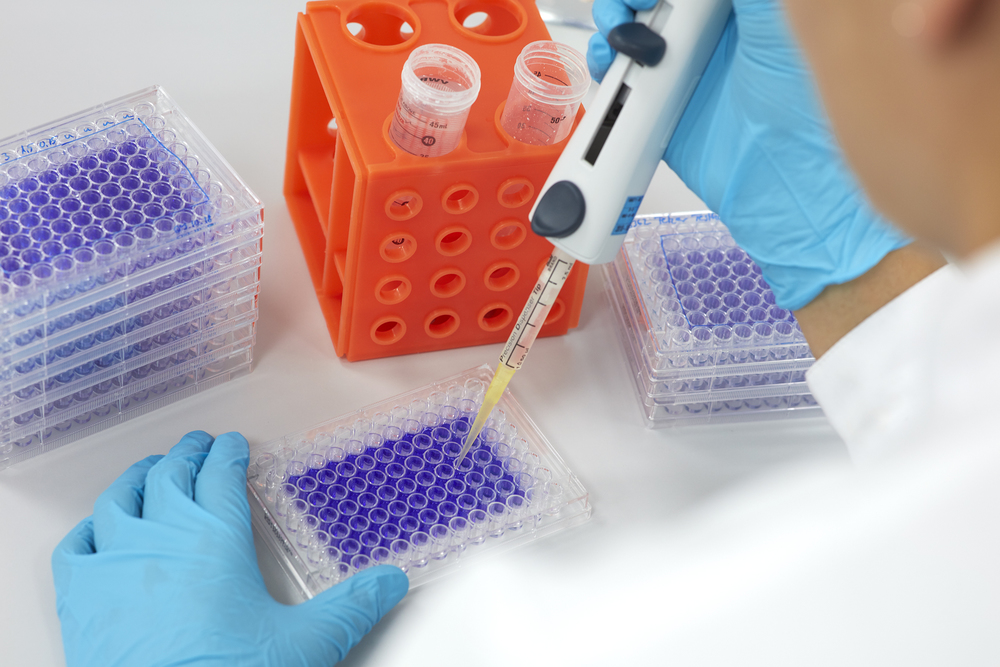
- In vitro cytotoxicity test acc. DIN EN ISO 10993-5
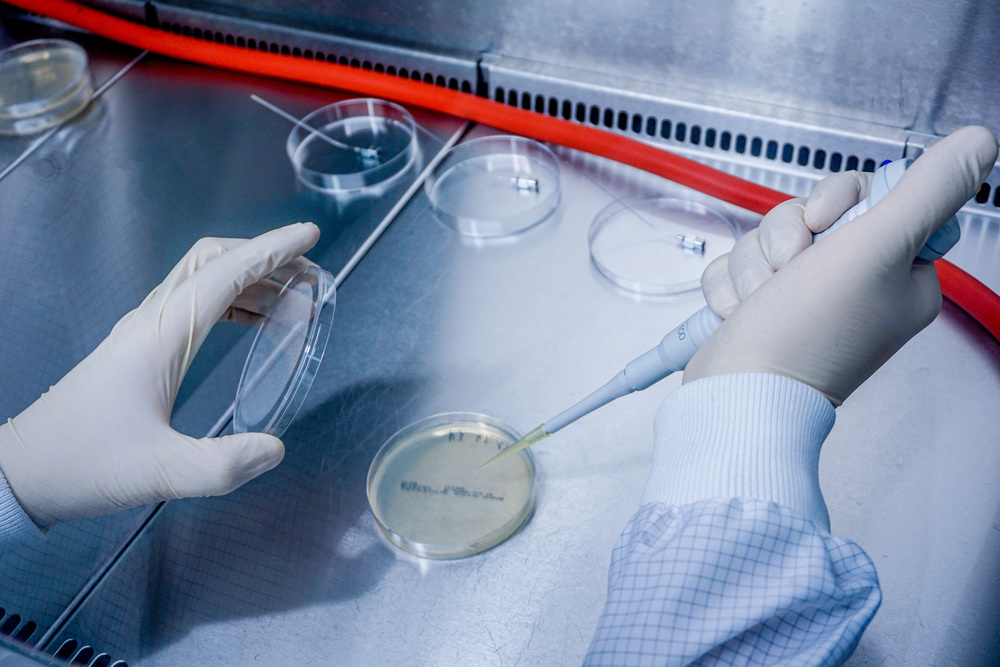
- Bioburden determination and its validation according to DIN EN ISO 11737-1
- Endotoxin test (LAL test) acc. USP 85 / Ph. Eur. 2.6.14
- Sterility check according to A EN ISO 11737-2
- Evaluation of contact plates for microbiological environmental monitoring
Particulate contamination testing
- using an optical particle counter (OPZ) based on USP 788 or
- microscopic evaluation according to VDI 2083 sheet 21 *, DIN EN ISO 16232 * or USP 788.
Validation of preparation and sterilization processes
Tests as part of the validation of the manufacturer's information in reprocessing instructions for reprocessing and sterilization processes of medical devices according to DIN EN ISO 17664 and ISO 17665-1 for
- the manual or mechanical reprocessing of medical devices
- steam sterilization of medical devices
- Simulation of reprocessing cycles of medical devices *
Validation of the final cleaning
Tests as part of the validation of the final cleaning of medical devices
- In vitro cytotoxicity test acc. DIN EN ISO 10993-5
- Determination of particulate contaminants based on USP 788
- TOC and THC determination *
- Bioburden determination acc. DIN EN ISO 11737-1
- Endotoxin test (LAL test)
Biocompatibility of medical devices
Tests to determine the biocompatibility of medical devices according to the amendment to DIN EN ISO 10993-1
- In vitro cytotoxicity test acc. DIN EN ISO 10993-5
- Sample preparation for the biological and chemical assessment of medical devices according to DIN EN ISO 10993-12
- Chemical characterization acc. DIN EN ISO 10993-18: GC-MS * / THC * / ICP-MS * / LC-MS * / Headspace *
- Brief toxicological assessment *
- Toxicological / biological evaluation of chemical analyzes of medical devices according to DIN EN ISO 10993-17 *
Products
For testing and monitoring the hygienic environmental cleanliness, we offer you products for microbiological-hygienic monitoring.
- Contact plates and sedimentation plates for microbiological and hygienic environmental monitoring
- Sampling bag for hygienically improved handling of samples when taking samples
After your sampling and sending to our laboratory, the samples will be incubated and evaluated in a defined manner.
* This service does not fall under the scope of the accreditation
CleanControlling Medical GmbH & Co. KG > Zertifikate
DIN EN ISO 17025:2018> Diese Kategorien könnten Sie interessieren

CleanControlling Medical GmbH & Co. KG
Gehrenstraße 11a78576 Emmingen-Liptingen
Deutschland
Fon: +49 7465 929678-0